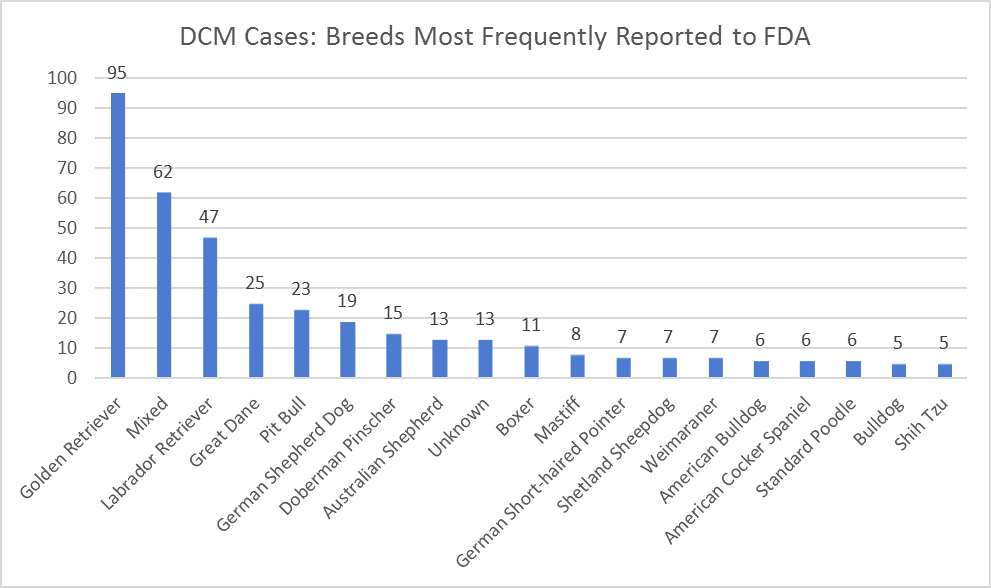
Product code: Fda 2025 heart disease
WHAT IS HEART DISEASE 2025, HEART DISEASE 2025, FDA Minority Health and Health Equity on X 2025, FDA Approves First Anti Inflammatory Drug for Heart Attack and 2025, FDA Investigation into Potential Link between Certain Diets and 2025, U.S. FDA Approves First Anti Inflammatory Drug for Cardiovascular 2025, FDA Investigation into Potential Link between Certain Diets and 2025, NEWS FDA Reports Some Dog Foods May Cause Heart Disease 2025, Heart Disease FDA 2025, FDA Dog Food Notice Veterinarian in Glastonbury CT 2025, Low dose colchicine gets FDA approval for secondary prevention of 2025, BioSpace FDA Clears Eko s Heart Disease Detection AI for Adults Ped 2025, After FDA Miss in Diabetes Lexicon Lands Long Awaited Approval in 2025, US FDA grants Fast Track designation to Angionetics Generx 2025, Alnylam gives up on expanded use of heart disease drug in US after 2025, How helpful was the FDA update on dog heart disease in choosing 2025, FDA Investigation into Potential Link between Certain Diets and 2025, FDA approves treatments for heart failure caused by rare disease 2025, Fda Dog Food And Heart Disease Store www.asdonline 1702351940 2025, FDA Approves Ancient Anti Inflammatory Drug for Heart Disease 2025, Ultromics receives FDA clearance for AI diagnostic for heart 2025, FDA Approves the Optimizer Smart Implantable Pulse Generator for 2025, How helpful was the FDA update on dog heart disease in choosing 2025, The FNIH Announces New Research Initiative to Identify More 2025, FDA pet food investigation What to know about diet related heart 2025, FDA Clears AI Heart Failure Detection System Science and Enterprise 2025, FDA warns of possible link between food canine heart disease 2025, FDA greenlights Numares Health s lipoprotein test for heart 2025, FDA approves novel treatments for heart disease 2025, FDA Updates on Heart Disease in Dogs Hemopet 2025, Interventional treatment for heart failure receives FDA approval 2025, In the fascinating world of heart failure treatment SGLT2 2025, FDA adcomm votes down Cytokinetics heart failure drug pharmaphorum 2025, FDA Grants Orphan Drug Designation to Mesoblast s Revascor for 2025, Omecamtiv Mecarbil for Heart Failure Turned Down by FDA MedPage 2025, FDA Approves Lexicon s INPEFA sotagliflozin Daily Pill For 2025, Dr. Jean Dodds Pet Health Resource Blog Dodds Responds to FDA 2025, FDA halts gene therapy for rare heart disease 2025, FDA approves Bristol Myers oral heart disease drug Health News 2025, Gout medication gets FDA approval to treat heart disease on back 2025, FDA contributes to hunt for new heart failure endpoints Fierce 2025, FDA casts doubt on Alnylam s Pfizer challenger in ATTR CM 2025, Cleerly Receives FDA Breakthrough Device Designation for Heart 2025, FDA names 16 brands of dog food linked to canine heart disease 2025, Shockwave Medical gets FDA nod for intravascular lithotripsy for 2025, FDA Approves New Bristol Myers Drug for Common Inherited Heart 2025, Zebra Medical gains FDA approval for AI powered heart disease 2025, 8 Ways Women Can Slash Their Heart Disease Risk FDA Says Best Life 2025, FDA Approves First in the World Device to Treat Congenital Heart 2025, FDA Approves Jardiance for HFpEF Heart Failure GoodRx 2025.
WHAT IS HEART DISEASE 2025, HEART DISEASE 2025, FDA Minority Health and Health Equity on X 2025, FDA Approves First Anti Inflammatory Drug for Heart Attack and 2025, FDA Investigation into Potential Link between Certain Diets and 2025, U.S. FDA Approves First Anti Inflammatory Drug for Cardiovascular 2025, FDA Investigation into Potential Link between Certain Diets and 2025, NEWS FDA Reports Some Dog Foods May Cause Heart Disease 2025, Heart Disease FDA 2025, FDA Dog Food Notice Veterinarian in Glastonbury CT 2025, Low dose colchicine gets FDA approval for secondary prevention of 2025, BioSpace FDA Clears Eko s Heart Disease Detection AI for Adults Ped 2025, After FDA Miss in Diabetes Lexicon Lands Long Awaited Approval in 2025, US FDA grants Fast Track designation to Angionetics Generx 2025, Alnylam gives up on expanded use of heart disease drug in US after 2025, How helpful was the FDA update on dog heart disease in choosing 2025, FDA Investigation into Potential Link between Certain Diets and 2025, FDA approves treatments for heart failure caused by rare disease 2025, Fda Dog Food And Heart Disease Store www.asdonline 1702351940 2025, FDA Approves Ancient Anti Inflammatory Drug for Heart Disease 2025, Ultromics receives FDA clearance for AI diagnostic for heart 2025, FDA Approves the Optimizer Smart Implantable Pulse Generator for 2025, How helpful was the FDA update on dog heart disease in choosing 2025, The FNIH Announces New Research Initiative to Identify More 2025, FDA pet food investigation What to know about diet related heart 2025, FDA Clears AI Heart Failure Detection System Science and Enterprise 2025, FDA warns of possible link between food canine heart disease 2025, FDA greenlights Numares Health s lipoprotein test for heart 2025, FDA approves novel treatments for heart disease 2025, FDA Updates on Heart Disease in Dogs Hemopet 2025, Interventional treatment for heart failure receives FDA approval 2025, In the fascinating world of heart failure treatment SGLT2 2025, FDA adcomm votes down Cytokinetics heart failure drug pharmaphorum 2025, FDA Grants Orphan Drug Designation to Mesoblast s Revascor for 2025, Omecamtiv Mecarbil for Heart Failure Turned Down by FDA MedPage 2025, FDA Approves Lexicon s INPEFA sotagliflozin Daily Pill For 2025, Dr. Jean Dodds Pet Health Resource Blog Dodds Responds to FDA 2025, FDA halts gene therapy for rare heart disease 2025, FDA approves Bristol Myers oral heart disease drug Health News 2025, Gout medication gets FDA approval to treat heart disease on back 2025, FDA contributes to hunt for new heart failure endpoints Fierce 2025, FDA casts doubt on Alnylam s Pfizer challenger in ATTR CM 2025, Cleerly Receives FDA Breakthrough Device Designation for Heart 2025, FDA names 16 brands of dog food linked to canine heart disease 2025, Shockwave Medical gets FDA nod for intravascular lithotripsy for 2025, FDA Approves New Bristol Myers Drug for Common Inherited Heart 2025, Zebra Medical gains FDA approval for AI powered heart disease 2025, 8 Ways Women Can Slash Their Heart Disease Risk FDA Says Best Life 2025, FDA Approves First in the World Device to Treat Congenital Heart 2025, FDA Approves Jardiance for HFpEF Heart Failure GoodRx 2025.